The carcinoid syndrome drug market is experiencing steady growth due to the increasing incidence of neuroendocrine tumors (NETs) and advancements in targeted therapies. Carcinoid syndrome is a rare condition caused by hormone-secreting carcinoid tumors, leading to symptoms like flushing, diarrhea, wheezing, and heart complications. Treatment includes somatostatin analogs (SSAs), targeted therapies, and symptomatic relief medications. Growing awareness of rare cancers and the expansion of oncology research are key factors driving market growth.
Market Size and Share
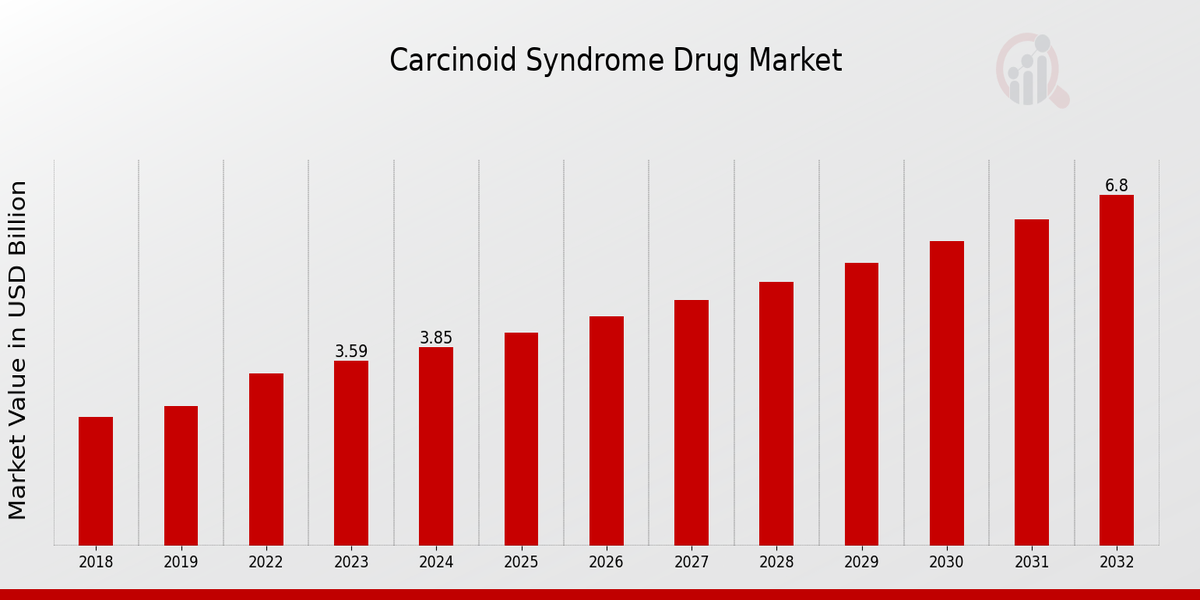
The global Carcinoid Syndrome Drug Market Size was estimated at 4.13 (USD Billion) in 2024. The Carcinoid Syndrome Drug Market Industry is expected to grow from 4.44 (USD Billion) in 2025 to 8.41 (USD Billion) till 2034, at a CAGR (growth rate) is expected to be around 7.36% during the forecast period (2025 - 2034). North America dominates the market due to strong research funding, high awareness levels, and robust healthcare infrastructure. The Asia-Pacific region is emerging as a fast-growing market due to increased healthcare investments and improvements in early diagnosis techniques.
Market Drivers
- Rising Incidence of Neuroendocrine Tumors (NETs): Increasing cases of gastrointestinal and lung neuroendocrine tumors.
- Advancements in Targeted Therapies: Development of somatostatin analogs (SSAs) and peptide receptor radionuclide therapy (PRRT).
- Growing Research in Rare Cancer Treatments: Increased clinical trials for novel carcinoid syndrome therapies.
- Favorable Regulatory Approvals for New Drugs: Fast-track approvals for orphan drugs targeting carcinoid syndrome.
Challenges and Restraints
- High Cost of Carcinoid Syndrome Treatments: Expensive somatostatin analogs and PRRT therapies.
- Limited Awareness in Developing Regions: Late diagnosis leads to poor disease management.
- Side Effects of Carcinoid Syndrome Medications: Issues like gastrointestinal disturbances and cardiovascular complications.
Market Trends
- Increased Adoption of Peptide Receptor Radionuclide Therapy (PRRT): Enhanced targeted treatment for advanced NETs.
- Development of Next-Generation Somatostatin Analogs: Focus on long-acting formulations for better symptom control.
- Advancements in Personalized Medicine: Expansion of biomarker-based therapies for carcinoid syndrome.
- Collaboration Between Biopharma Companies: Research into novel NET-targeting compounds.
Regional Analysis
- North America: Leading market due to high awareness, strong R&D funding, and advanced oncology treatment centers.
- Europe: Growing adoption of novel targeted therapies for NET-related disorders.
- Asia-Pacific: Rapid growth due to improved diagnostic capabilities and rising cancer incidence.
- Rest of the World: Increasing healthcare investments in oncology and rare disease treatments.
Segmental Analysis
- By Drug Type:
- Somatostatin Analogs (SSAs) (e.g., Octreotide, Lanreotide, Pasireotide)
- Targeted Therapies (e.g., Everolimus, Sunitinib)
- Peptide Receptor Radionuclide Therapy (PRRT) (e.g., Lutetium-177-DOTATATE)
- Symptomatic Relief Drugs (e.g., Telotristat Ethyl for Diarrhea Control)
- By Route of Administration:
- Oral
- Injectable
- By Distribution Channel:
- Hospital Pharmacies
- Specialty Clinics
- Online Pharmacies
Key Market Players
- Sanofi
- Merck and Co
- Ipsen
- Eisai
- HoffmannLa Roche
- Celgene
- TetraLogic Pharmaceuticals
Recent Developments
- Expansion of Clinical Trials for New Carcinoid Syndrome Therapies: Exploring novel SSA formulations.
- FDA and EMA Approvals for Next-Generation PRRT Treatments: Advancements in radionuclide therapy for NETs.
- Increased Investment in Rare Cancer Drug Development: Focus on orphan drug research for carcinoid syndrome.
For more information, please visit us at marketresearchfuture.