IMARC Group, a leading market research company, has recently released a report titled “Preclinical CRO Market Report by Service (Bioanalysis and DMPK Studies, Toxicology Testing, and Others), End Use (Biopharmaceutical Companies, Government and Academic Institutes, Medical Device Companies), and Region 2024-2032”. The study provides a detailed analysis of the industry, including the preclinical CRO market share, trends, size, and industry trends forecast. The report also includes competitor and regional analysis and highlights the latest advancements in the market.
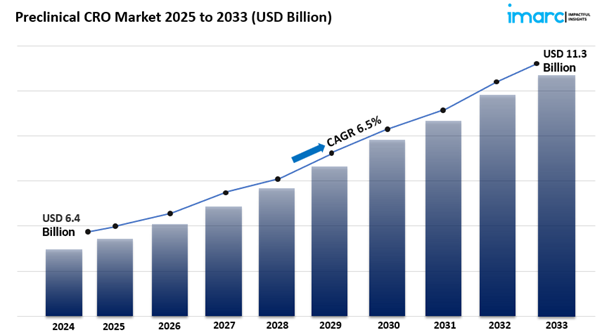
The global preclinical CRO market size reached USD 6.4 Billion in 2024. Looking forward, IMARC Group expects the market to reach USD 11.3 Billion by 2033, exhibiting a growth rate (CAGR) of 6.5% during 2025-2033.
Request to Get the Sample Report:
https://www.imarcgroup.com/preclinical-cro-market/requestsample
Preclinical CRO Market Trends
The preclinical CRO market is set for significant growth. It will adapt to the needs of the pharmaceutical and biotechnology sectors. In 2023, the demand for outsourced research will boost growth. Companies will seek cost-effective and efficient preclinical solutions. By 2024, technological advancements will be key. Innovations in AI, high-throughput screening, and modeling will enhance research.
Moreover, the focus on regulatory compliance will increase. CROs will rank according to guidelines to build client trust. As these trends combine, the market will grow and evolve. It will highlight the need for innovation, compliance, and partnerships. Advanced technologies and regulatory excellence will be crucial for success.
Market Dynamics 1: Rising Demand for Outsourced Research Services
The preclinical Contract Research Organization (CRO) market is booming. Pharmaceutical and biotech firms are outsourcing more to speed up drug development. As preclinical studies grow more complex, these firms seek CROs for their expertise, resources, and technology. By 2024, this trend will likely grow. The drive? A need for cheaper, faster solutions. Outsourcing lets companies focus on what they do best. They gain access to high-quality data and expert knowledge from CROs. The push for innovation in drug discovery is also key. This is especially true for biologics and personalized medicine. This need for tailored services from CROs is vital. Thus, the preclinical CRO market is set for strong growth. Companies are forming partnerships to boost research and productivity.
Market Dynamics 2: Tech Advances in Preclinical Research
Tech advances are reshaping the preclinical CRO market. They make research faster and more accurate. Innovations like high-throughput screening, in vitro models, and better imaging are key. By 2024, we expect more use of AI and machine learning. These will improve data analysis and predictions. Such technologies also cut drug development time and costs.
Moreover, organ-on-a-chip and 3D bioprinting technologies are emerging. They offer better models for testing, making results more applicable to clinical settings. As CROs adopt these technologies, they will meet client demands better. This, in turn, will boost the preclinical CRO market.
Market Dynamics 3: Regulatory Changes and Compliance Requirements
The preclinical CRO market experiences significant impacts from regulatory changes. These changes impact how researchers conduct studies. Regulatory bodies now demand strict testing and validation for new drugs. This has increased the scrutiny of preclinical data.
By 2024, CROs must follow the latest guidelines to stay credible and shift to clinical trials. This need pushes them to invest in quality assurance and regulatory expertise. They aim to handle compliance complexities. As data integrity standards tighten, CROs must adopt strong data management systems. This ensures their results are accurate and reliable. Focusing on compliance boosts the trust in their studies. It also makes CROs more appealing to drug companies. This increases demand for their services.
Preclinical CRO Market Report Segmentation:
By Service:
- Bioanalysis and DMPK Studies
- Toxicology Testing
- Others
Toxicology testing accounted for the largest market share as it is essential for ensuring the safety of drug candidates before clinical trials, making it a critical component of preclinical studies.
By End Use:
- Biopharmaceutical Companies
- Government and Academic Institutes
- Medical Device Companies
Biopharmaceutical companies represented the largest segment as they rely on preclinical CROs to streamline drug development processes and reduce costs.
Regional Insights:
- North America
- Asia-Pacific
- Europe
- Latin America
- Middle East and Africa
North America's dominance in the preclinical CRO market is attributed to its advanced healthcare infrastructure, high research, and development (R&D) spending, and the presence of numerous leading pharmaceutical and biotechnology companies.
Competitive Landscape with Key Players:
The competitive landscape of the preclinical CRO market size has been studied in the report with the detailed profiles of the key players operating in the market.
Some of These Key Players Include:
- Charles River Laboratories Inc.
- Covance Inc. (Laboratory Corporation of America Holdings)
- Eurofins Scientific
- ICON Plc
- MD Biosciences Inc. (MLM Medical Labs)
- Medpace
- Parexel International Corporation
- PPD Inc.
- Wuxi AppTec
Ask Analyst for Customized Report:
https://www.imarcgroup.com/request?type=report&id=3715&flag=C
Key Highlights of the Report:
- Market Performance (2018-2023)
- Market Outlook (2024-2032)
- Market Trends
- Market Drivers and Success Factors
- Impact of COVID-19
- Value Chain Analysis
If you need specific information that is not currently within the scope of the report, we will provide it to you as a part of the customization.
About Us
IMARC Group is a leading market research company that offers management strategy and market research worldwide. We partner with clients in all sectors and regions to identify their highest-value opportunities, address their most critical challenges, and transform their businesses.
IMARC’s information products include major market, scientific, economic and technological developments for business leaders in pharmaceutical, industrial, and high technology organizations. Market forecasts and industry analysis for biotechnology, advanced materials, pharmaceuticals, food and beverage, travel and tourism, nanotechnology and novel processing methods are at the top of the company’s expertise.
Contact Us:
IMARC Group
134 N 4th St
Brooklyn, NY 11249, USA
Website: imarcgroup.com
Email: [email protected]
Americas: +1-631-791-1145 | Europe & Africa: +44-753-713-2163 | Asia: +91-120-433-0800