Market Overview 2025-2033
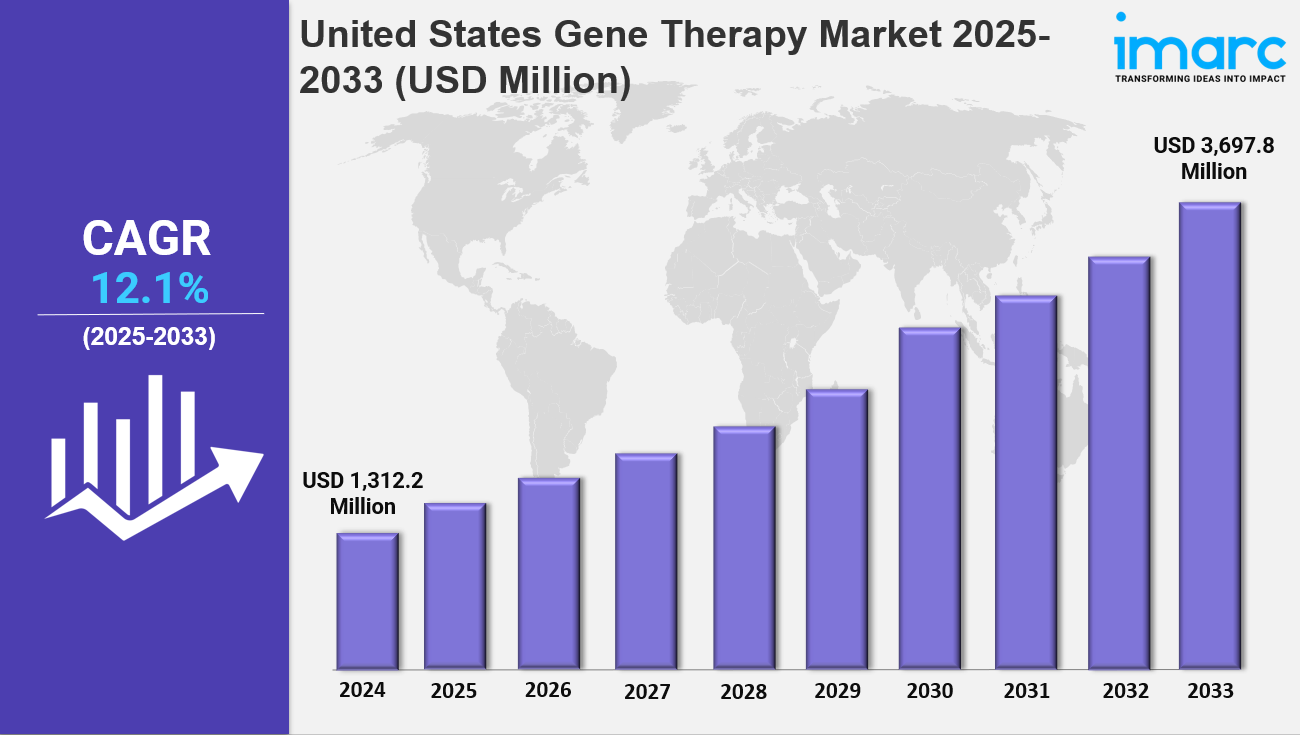
The United States gene therapy market size was valued at USD 1,312.2 Million in 2024. Looking forward, IMARC Group estimates the market to reach USD 3,697.8 Million by 2033, exhibiting a CAGR of 12.1% from 2025-2033. The market is witnessing significant expansion, fueled by advancements in genetic research, growing prevalence of genetic disorders, and rising investments in biotechnology. Key trends include the development of targeted therapies and personalized medicine, with leading companies emphasizing regulatory approvals and strategic collaborations to enhance treatment accessibility and innovation.
Key Market Highlights:
✔️ Strong expansion driven by advancements in genetic research & biotech innovation
✔️ Growing focus on personalized medicine and targeted gene therapies
✔️ Surge in clinical trials and FDA approvals boosting market confidence
Request for a sample copy of the report: https://www.imarcgroup.com/united-states-gene-therapy-market/requestsample
United States Gene Therapy Market Trends and Drivers:
The rise in rare genetic disorders is playing a pivotal role in transforming the United States Gene Therapy Market, as pharmaceutical companies and research institutions prioritize novel therapeutic solutions for conditions with limited treatment options. Gene therapy, which aims to treat or even cure these diseases by targeting the root cause at the genetic level, has gained significant traction. This demand has led to robust pipeline development, with several therapies in Phase III clinical trials.
In 2025, breakthroughs in CRISPR and viral vector technologies are expected to drive further innovation, especially for diseases such as spinal muscular atrophy, hemophilia, and Duchenne muscular dystrophy. The United States Gene Therapy Market Size is projected to reflect substantial growth as a result of these advancements, with government incentives and fast-track regulatory designations encouraging faster commercialization. Additionally, collaborations between biotech firms and academic centers have accelerated preclinical research and expanded the scope of treatable diseases, thereby reinforcing the long-term sustainability of the market.
The influx of capital into the gene therapy sector is significantly enhancing the United States Gene Therapy Market Share by enabling companies to expand their manufacturing capabilities and pursue complex R&D initiatives. Venture capital, public-private partnerships, and mergers and acquisitions are reshaping the competitive landscape, making it more dynamic and innovation-driven. In 2025, increased investor confidence, fueled by successful product launches and favorable reimbursement frameworks, is expected to propel the market even further.
Major pharmaceutical players are also entering strategic alliances with smaller biotech startups to gain access to proprietary gene-editing platforms and expand their therapeutic portfolios. As infrastructure and production technologies evolve, this will reduce the overall cost of gene therapies, improving patient access and broadening the commercial potential. These developments contribute significantly to the growth in the United States Gene Therapy Market Share, ensuring that both existing and new market entrants continue to play a role in shaping future trends.
The evolution of regulatory pathways is a critical driver behind the United States Gene Therapy Market Growth, as government agencies like the FDA streamline approval processes for gene-based treatments. Recent frameworks that support accelerated approval and orphan drug designation have shortened development timelines and enhanced investor and developer confidence. As of 2025, these regulatory enhancements are poised to further reduce market entry barriers and increase the pace of new treatment launches.
The agency’s commitment to post-market surveillance and long-term patient safety has also increased public trust in gene therapy, which is vital for market expansion. By providing clear guidance on manufacturing standards and clinical trial design, regulators are not only promoting innovation but also ensuring consistent quality and safety. This evolving support system is expected to fuel the United States Gene Therapy Market Growth by attracting new players, stimulating innovation, and encouraging broader therapeutic applications across oncology, neurology, and rare diseases.
Speak to An Analyst: https://www.imarcgroup.com/request?type=report&id=11240&flag=C
United States Immunoassay Market Segmentation:
The market report segments the market based on product type, distribution channel, and region:
Study Period:
Base Year: 2024
Historical Year: 2019-2024
Forecast Year: 2025-2033
Analysis by Gene Type:
- Antigen
- Cytokine
- Tumor Suppressor
- Suicide Gene
- Deficiency
- Growth Factors
- Receptors
- Others
Analysis by Vector Type:
- Viral Vector
- Adenoviruses
- Lentiviruses
- Retroviruses
- Adeno-Associated Virus
- Herpes Simplex Virus
- Poxvirus
- nia ViruVaccis
- Others
- Non-Viral Techniques
- Naked and Plasmid Vectors
- Gene Gun
- Electroporation
- Lipofection
- Others
Analysis by Application:
- Oncological Disorders
- Rare Diseases
- Cardiovascular Diseases
- Neurological Disorders
- Infectious Disease
- Others
Regional Insights:
- Northeast
- Midwest
- South
- West
Competitive Landscape:
The market research report offers an in-depth analysis of the competitive landscape, covering market structure, key player positioning, top winning strategies, a competitive dashboard, and a company evaluation quadrant. Additionally, detailed profiles of all major companies are included.
Contact Us:
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: [email protected]
Tel No:(D) +91 120 433 0800
United States: +1-631-791-1145

